Activated carbon is a crude form of graphite. The difference with graphite is in the amount of fine distributed pores and the very large internal pore surface. Activated carbon is very porous with a random imperfect structure with many pores. The graphite structure in the active carbon gives rise to a very large surface area that permits the adsorption of a large number of compounds.
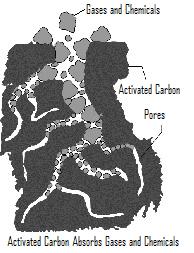 Activated carbon has the strongest physical adsorption and the largest adsorption pore volume of all known materials.
Activated carbon has the strongest physical adsorption and the largest adsorption pore volume of all known materials.
Activated carbon can have a surface area greater than 1,000 m² / g. This means that 3 g of activated carbon, have the surface area of a soccer field.
Activated carbon is made by using heat to remove gases and fibrous impurities from materials such as bone meal, peanut shells, fruit seeds, peat, coconut, coal or wood.
The size of the set of pores depends on the raw material used and the method of activation.
Coconut provides a highly micro porous activated carbon, used in filters for air purification. Wood gives a macro porous activated carbon, which is suitable for the removal of large molecules from fluids, suitable for water purification.
Activated carbon also has applications in medicine for adsorption of toxins and to decolorize and deodorize.
Also known Norit tablets for diarrhea include activated carbon.
