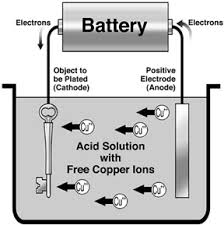
 Electroplating is the electrochemical covering with a layer of metal (usually zinc, nickel, chromium) to protect against corrosion (and embellishment). The basic principle of electroplating is the reverse of the operation of a battery or battery pack. A salt of the metal covering (e.g. zinc chloride) is dissolved in water and decomposes into ions. The object is as a cathode connected to the - terminal. (The power thus sends extra electrons). Another metal is connected to the positive as anode and immersed. The dissolved metal ions react and deposit on the cathode.
Electroplating is the electrochemical covering with a layer of metal (usually zinc, nickel, chromium) to protect against corrosion (and embellishment). The basic principle of electroplating is the reverse of the operation of a battery or battery pack. A salt of the metal covering (e.g. zinc chloride) is dissolved in water and decomposes into ions. The object is as a cathode connected to the - terminal. (The power thus sends extra electrons). Another metal is connected to the positive as anode and immersed. The dissolved metal ions react and deposit on the cathode.
Nickel gives a very tight protection.
Chromium is widely used for plumbing, and was used for cars and bikes. By combining with underlying nickel it shines.
Zinc is usually combined with iron or nickel in order to avoid zinc oxide.
Copper is mainly used for printed circuit boards, tin on steel.
Galvanizing goes also thermally
The workpiece is in hot bath first alkaline degreased and then stained with hydrochloric acid.
Then, it goes through a solution of salt and water (ZnCl2 . 2NH4Cl) and then in molten zinc at a temperature of ± 450°C. This results in an alloy.
