From a pure substance can only arise another substance by a (chemical) reaction.
Substances can occur in three phases or states of matter, such as water: solid (ice), liquid or gas (steam) (and 4: plasma). The relationship between the molecules is then changed by temperature and / or pressure. Intermolecular forces are broken or formed, but the composition of the substance is retained. Transition from one form to another: Gas: condensation<vaporization/> ; liquid: > solidification / < melt; solid: < sublimate / deposition. (Gas –plasma: (de)ionization. 4thphase,plasma: ionized gas, the universeis full of it.Closer: Aurora, andball lightningpresumably also.)
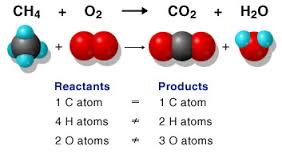 In a reaction are new compounds and fabrics made. E.g. in the combustion of methane (= oxidation with oxygen): CH4 + 2O2 > CO2 + 2H2O are carbon dioxide and hydrogen formed.
In a reaction are new compounds and fabrics made. E.g. in the combustion of methane (= oxidation with oxygen): CH4 + 2O2 > CO2 + 2H2O are carbon dioxide and hydrogen formed.
Oxidation is a chemical process in which a substance (the reducer, electron donor) emits electrons to another substance (the oxidizer) in which the oxidation number of the reducer increases.
It's (almost always) a reaction with oxygen that we know as burn or explode (fast) or rust (slow). With rust is only referred to the reaction of iron with oxygen. Oxidations of other metals are known as corrosion.
Oxidation is always associated with a reduction. The transferred electrons are actually to be captured by another substance (the oxidizer), which thus is reduced (they get more (negative) electrons). The whole (oxidation and reduction together) is called an oxidation-reduction reaction, or redox reaction.
Some reactions release energy (heat), other consume energy.
Often, reactions take place in two directions, until a chemical equilibrium is created.
The reaction rate is influenced by the concentration, temperature, degree of distribution (coarse - fine), and sometimes also electromagnetic radiation, the nature of the compounds, and a catalyst.
A catalyst lowers the energy needed to start a reaction but is not itself consumed during the reaction. The reaction then proceeds through intermediate reactions. (An enzyme is a biological catalyst.)
Electrolysis is a chemical reaction in which by an electric current complex substances (dissolved or melted) are decomposed into simple substances and / or other composite materials. It is applied by electroplating.
In electrolysis, it seems as if only reduction occurs. On the negative electrode this seems to be correct. On the cathode (the pole which emits electrons) is the metal created from its salt. For an electrolysis, however, are always necessary, two electrodes; and at the anode, an oxidation takes place.
An electrolyte is in accumulators and batteries, the medium that forms the connection between the two poles (anode and cathode) of an electrochemical energy source or cell.
Electrolytes are complex substances which split in a composite solution or in the molten state in whole or in part, into ions; and conduct the electric current.
