The meters high furnace, which approximately operates in the same manner as the puddling furnace, can reach a higher temperature so the iron can be melted, and drained at the base of the furnace.
The iron ore is free from residues and slag, but there is too much carbon in dissolved, about 4%. This cast iron was very hard, but brittle. It is suitable for some applications (casseroles..), but unusable, for example, for swords, and fast-moving machine parts.
For use in the blast furnace of the ore are first made lightly castable pebbles by sintering or pellet pressing, depending on the grain size of the base material.
Sintering
Ore is crushed into granules <6 mm and mixed with fine charcoal or coke and additives (e.g. limestone). This is ignited. This will cause the ore baking and stick together or sintering as porous mass. That is, after cooling, crushed in for the blast furnace suitable fragments (5-40 mm). Good gas permeability of the sintered ore in the blast furnace makes a rapid reduction reaction possible.
In the pelletizing is very finely ground ore powder (0.01 to 0.1 mm), (not suitable for sintering) mixed with water and clay, limestone, and the carbon powder. This mixture is kneaded in rotating drums to marbles of 10-30 mm diameter. Which are baked in a continuous oven, so that they have sufficient strength and porosity allowing them to be loaded into the blast furnace.
This ore preparation is needed in order to get a uniformly filling. It avoids clogging of the blast furnace and blowing of ore particles. It ensures a uniform product composition for the blast furnace which operates continuously for many years.
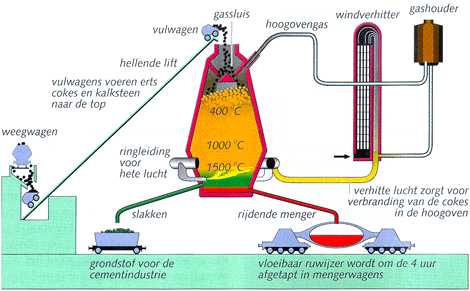
In a blast furnace from below hot air is blown. The hot air reacts with the coke (or pulverized coal), to CO and becomes much hotter. This CO reacts with the iron ore pellets or the sinter lumps which are added from the top. While the mass falls, the ore is increasingly reduced (removal of oxygen: Fe2O3 to Fe3O4 to FeO), until liquid iron (Fe) is created at the bottom.
At the ore limestone was added to bind present silicates to liquid slag. The slag floats on the heavier pig iron. They are separated and drained as a granulated raw material for cement.
The gases are collected, purified, and used for pre-heating of the air input.
At the bottom the drops of hot metal (pig iron) are collected. This is processed to steel. In order to do this, the hot steel product is to be hammered a longer time, so that the greater part of the carbon may be burned to the air.
That was so cumbersome that they often chose to prepare it from wrought iron that was produced in a puddling furnace.
Increased knowledge of chemistry taught what substances they had to add to catch undesirable components (e.g. too much sulfur or phosphorus) into fabrics that blend with the slag, so a high quality iron could be produced.
To improve the quality of the hot iron it was used to stir in a half-open “puddle furnace' with long (consumed) rods, so that the carbon could escape through the air. The product deserves already the name 'steel '. It was suitable for plowing, weapons...
Later steel was made in the Bessemer process in a converter. The excess of carbon was oxidized by blowing air through the molten pig iron. The carbon also serves as a further fuel. If the process was once going, it kept itself working. So it was a very economical process.
The Bessemer process was supplanted by the flame furnace in which pig iron, iron ore and scrap were melted in such proportions that most carbon and oxygen escaped as carbon monoxide. With this gas, the flow of air was then pre-heated.
