Calcium oxide is quick lime (burnt lime, lime, CaO...), a white powder.
It is prepared by heating calcium carbonate ((CaCO3; mineral calcite) e.g. limestone, but also (oyster) shells, (egg) shells,..), in a special kiln (rotary kiln) to about 900°C, so it decomposes in oxide and carbon dioxide.
This is called calcination or lime- burning.
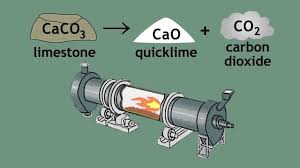 The production of calcium oxide is one of the oldest chemical processes that are applied by the human being, due to the abundance of limestone in the earth's crust and the convenience of the transformation into calcium oxide. Its use predates recorded history.
The production of calcium oxide is one of the oldest chemical processes that are applied by the human being, due to the abundance of limestone in the earth's crust and the convenience of the transformation into calcium oxide. Its use predates recorded history.
Alternate layers of coal dust (or other fuel) and shells (or marl..) are deposited on top of the lime kilns.
After approximately 24 hours of heating, were at the bottom of the oven, the shells then removed. They are quenched with water: 2 buckets of water over a wheelbarrow shells. Then the shells are dug and an extra bucket of water added. This creates a chemical reaction that makes a big smoke. The shells disintegrate into fine powder lime. The lime is then sieved.
The quicklime is not stable and will spontaneously react with CO2 from the air until it is converted again entirely into calcium carbonate.
Air lime or (high) calcium lime, and hydraulic lime
Lime quenching is done with water (wet quenching) or wet sand (dry quenching). In one case, the dry lime powder occurs, in the other a wet lime dough or putty. (CaO + H2O -> Ca ( OH)2)
Difference between air lime, lime plaster (powder) or hydrated lime, and hydraulic lime.
Air lime is made of (almost) pure limestone (CaO). Which cures by taking CO2 from the air, making it rock-hard calcium carbonate again. The carbonation begins when the mortar dries which gives carbon dioxide from the air access to the limestone. (Not too dry, the reaction CO2 + H2O -> H2CO3 also needs some water. It can therefore take a long time before the inner cores are cured and hard. The micro - pore structure of hardened hydrated lime is much finer.
Hydrated lime can’t be used as the binder in mortar because it never gets hard. It dries to air.
If the raw material consists of limestone with clay (SO3, and minerals), creates hydraulic lime. The clay is converted to calcium aluminates and silicates during the burning process .This makes an air hardening lime share and a water-reactive hydraulic component that in a chemical reaction becomes hard, different from just drying out in air.
Hydraulic lime can be used as a binder for mortar.
Calcium silicates and other oxides will react with water to form gels and which later form structures that solidify and harden. Through this combined reaction (hydration and carbonation) a tough product is creates that can capture deformations in constructions.
Applications with lime
When lime is mixed with water and sand you get (lime) mortar.
Other building products based on lime are lime plaster and Portland cement.
If lime is heated with quartz sand (SiO2) and sodium carbonate (Na2CO3) glass is produced.
If lime is mixed with molten ore (iron) I forms slag which is not miscible with the molten iron, so that the silicates can be removed from the iron. Approximately 80 kg of lime is used for the production of every ton of iron.
Lime is also used in the production of other metals. For example, to remove silicates from aluminum oxide (Al2O3).
The paper making uses it in the pulp, because lime is very alkaline, and dissolves the lignin (wood fibers that hold the wood together).
Before the advent of electric lighting white stage lighting was made by heating limestone in the flame of a torch: the first spotlight.
The high melting point makes it attractive as a refractory material, such as in the lining of furnaces.
Other applications:
To remove hair of animal skins
cleaning of waste water
to accelerate the breakdown of cadavers
in agriculture and horticulture as lime fertilizer,
and in order to warm up military rations. This takes place without flames, by the exothermic reaction with water. The volume doubles while about. You can be badly burned.
